how many electrons are in radon|Radon (Rn) : Clark MedicalAn early-20th-century form of quackery was the treatment of maladies in a radiotorium. It was a . Tingnan ang higit pa #331 of 1,190 Restaurants in Makati 5 reviews. Second Floor, Ayala North Exchange Ayala Avenue Corner Amorsolo Street, Legaspi Village. 0 km from Greenbelt Mall . Esperanza Street Corner Makati Avenue New World Makati Hotel, Ayala Center. 0.2 km from Greenbelt Mall
PH0 · WebElements Periodic Table » Radon » the essentials
PH1 · Radon Element Facts
PH2 · Radon (Rn)
PH3 · Radon
PH4 · Complete Electron Configuration of Radon (Rn)
PH5 · Chemical Elements.com
I5 6400 support h61 Response: The Intel i5-6400 processor uses different socket, and is not supported by the Intel H61 chipset. mother compatibly . 2021-08-12 01:26:06. . Socket: FCLGA1155, 1155 LGA RAM: 8GB HDD's: 1x 1TB and 1x 250GB I plan eventually to replace the HDD's to 2x 1TB SSD's to already have some increase in read- and write .
how many electrons are in radon*******Radon is a chemical element; it has symbol Rn and atomic number 86. It is a radioactive noble gas and is colorless and odorless. Of the three naturally occurring radon isotopes, only radon-222 has a sufficiently long half-life (3.825 days) for it to be released from the soil and rock where it is . Tingnan ang higit paPhysical propertiesRadon is a colorless, odorless, and tasteless gas and therefore is not detectable by human senses alone. At standard . Tingnan ang higit pa
MedicalAn early-20th-century form of quackery was the treatment of maladies in a radiotorium. It was a . Tingnan ang higit paIn minesRadon-222 decay products have been classified by theSince . Tingnan ang higit pa• Radon at the United States Environmental Protection Agency• National Radon Program Services hosted by Tingnan ang higit pa
Radon was discovered in 1899 by Ernest Rutherford and Robert B. Owens at McGill University in Montreal. It was the fifth radioactive element to be discovered, after uranium, . Tingnan ang higit paConcentration unitsAll discussions of radon concentrations in the environment refer to Rn. While the average rate of production of Rn (from the thorium . Tingnan ang higit pa
• Chemistry portal• International Radon Project• Lucas cell• Pleochroic halo (aka: Radiohalo) Tingnan ang higit pa
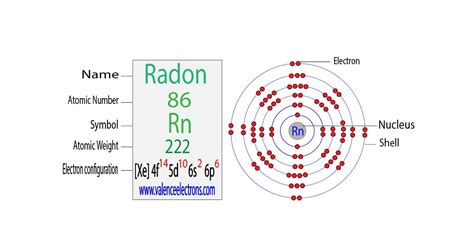
The atomic number of each element increases by one, reading from left to right. BlockElements are organised into blocks by the orbital type in which the outer electrons . The total number of electrons in radon is eighty-six. These electrons are arranged according to specific rules in different orbitals. The arrangement of electrons in . Radon atoms possess a particularly stable electronic configuration of eight electrons in the outer shell, which accounts for the characteristic chemical inactivity of the element. Radon, however, is .Rn. (222) The chemical element radon is classed as a noble gas and a nonmetal. It was discovered in 1900 by Fredrich E. Dorn. Data Zone. Show more, including: Heats, Energies, Oxidation, Reactions, .Atomic Number: 86. Atomic Mass: (222.0) amu. Melting Point: -71.0 °C (202.15 K, -95.8 °F) Boiling Point: -61.8 °C (211.35 K, -79.24 °F) Number of Protons/Electrons: 86. Number of Neutrons: 136. Classification: .Radon (Rn) has an atomic mass of 86. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more.Radon atoms have 86 electrons and the shell structure is 2.8.18.32.18.8. The ground state electronic configuration of neutral radon is [ Xe ]. 4f 14 . 5d 10 . 6s 2 . 6p 6 and the term symbol of radon is 1 S 0 .History. Radon was discovered in 1900 by Friedrich Ernst Dorn in Halle, Germany. He reported some experiments in which he noticed that radium compounds emanate a radioactive gas. In 1910, Sir William Ramsay .Electron configuration for radon. The history of Radon. Periodic table history. Identifiers. List of unique identifiers for Radon in various chemical registry databases. Radon is a . Radon has 86 protons and electrons; the number of neutrons is different for each isotope: Number of neutrons = Atomic Mass of the Rn isotope - 86The numbers of protons in an ion of radon are also two, eight, eighteen, thirty-two, eighteen and eight in the six protons shells of radon. There are 86 protons and electrons in radon. There are 86 electrons in radon and there are also 86 protons in radon. FUN FACT: There are 136 neutrons in radon. Radon is a chemical element which has chemical symbol Rn. The atomic number of radon is 86. It is a colourless, radioactive, tasteless, odourless noble gas. It occurs naturally found in minute .how many electrons are in radon Radon (Rn) What is the number of protons neutrons and electrons of radon? There are 88 protons/electrons in radium. There are 138 neutrons. the amount of protons is 86 and the amount of electrons is 96 and . In the isotope Radon-220 (Rn-220), there are 134 neutrons. This can be determined by subtracting the atomic number of Radon (86) from the mass number of Rn-220, which is 220. Radon is a non metal .
Radon is a chemical element of the periodic table with chemical symbol Rn and atomic number 86 with an atomic weight of 222 u and is classed as a noble gas. . Electron shell for Radon, created by Injosoft AB Rn. Figure: Shell diagram of Radon (Rn) atom. Orbital Diagram. 1s: 2s: 2p: 3s: 3p: 3d: 4s: 4p: 4d: 4f: 5s: 5p: 5d: 6s: 6p: The history . Protons. A proton is one of three main particles that make up the atom. Protons are found in the nucleus of the atom. This is a tiny, dense region at the center of the atom. Protons have a positive electrical charge of one ( + 1) and a mass of 1 atomic mass unit (amu), which is about 1.67 × 10 − 27 kilograms.Valence electrons are the electrons present in the outermost shell of an atom. You can easily determine the number of valence electrons an atom can have by looking at its Group in the periodic table. For example, atoms in Groups 1 and 2 have 1 and 2 valence electrons, respectively. Atoms in Groups 13 and 18 have 3 and 8 valence electrons .Radon (Rn) Hence the Radon element has electrons arrangement 2, 8, 18, 32, 18, 8. . Radon has many isotopes and all those isotopes are radioactive in nature. Radon is a colorless gas at room temperature. But when it is cooled below its freezing point, it emits a light that changes from yellow to orange-red.
A step-by-step description of how to write the electron configuration for Radon (Rn).In order to write the Rn electron configuration we first need to know th.
Radium ion (Ra 2+) electron configuration. The ground-state electron configuration of radium is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 4f 14 5s 2 5p 6 5d 10 6s 2 6p 6 7s 2. This electron .
how many electrons are in radonComplete Step by step solution: - We should understand the meaning of the term valence. It is basically the electrons in the valence shell of an atom called valence electrons. - As we know that the atomic number of radon is 6, and it is a noble gas. It is found in the 18 the column this means that radon will end in an electron configuration of .
In the ground state of Rn, the electrons are arranged following the classical rules of filling according to the increasing order of energies. Orbital Energy Picture of Rn Radon Condensed Electron Configuration. Radon condensed electron configuration is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 6. It is the . Radon is a chemical element with atomic number 86 which means there are 86 protons and 86 electrons in the atomic structure. The chemical symbol for Radon is Rn. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The nucleus is composed of protons and neutrons.

The element Radon was discovered by E. Rutherford and R. B. Owens in year 1899 in Germany. Radon was first isolated by W. Ramsay and R. Whytlaw-Gray in 1910. How many valence electrons does a Radon atom have? Radon has 6 valence electrons. Radon has 86 electrons out of which 6 valence electrons are present in the 6s2 6p6 .
A $ {}_{86}^{222}Rn{{ }} $ is an Isotope of noble gases radon. How many protons, Neutrons, and electrons are there in one atom of this radon isotope.. Ans: Hint: Isotopes are a species of atoms of an element with different atomic masses but the same.
Modern Chemistry. 1st Edition • ISBN: 9780547586632 (2 more) Jerry L. Sarquis, Mickey Sarquis. 2,184 solutions. 1 / 4. Find step-by-step Chemistry solutions and your answer to the following textbook question: How many protons and electrons are in each atom? a. radon b. magnesium. None. There are no unpaired electrons in a radon atom or any atom of the noble gases. The atomic number of radon is 76, which means that a neutral atom would also contain 76 electrons. The noble gas shorthand electron configuration is ["Xe"]4"f"^14"5d"^10"6s"^2"6p"^6". All of the energy sublevels are filled to the point of .
New York (Newark) (EWR) to. Manila (MNL) 20 Feb 25 (Thu) - 29 Mar 25 (Sat) From. USD 6,546* Seen: 8 hrs ago. Round-trip / Business. Book now. keyboard_arrow_right *Fares displayed have been collected within the last 48hrs and may no longer be available at the time of booking. Additional baggage fees and charges for optional products and .
how many electrons are in radon|Radon (Rn)